Clinical Trial Design
Phase I studies: we developed a novel dual-endpoint dose-finding design that incorporates an efficacy outcome and toxicity data with multiple types over multiple treatment cycles. This is joint work with Dr. Jun Yin and Dr. Sumithra Mandrekar from Mayo Clinic.
Phase II studies: we proposed and evaluated a two-stage, Phase II, adaptive clinical trial design. Its goal is to determine whether future Phase III (confirmatory) trials should be conducted, and if so, which population should be enrolled (Advised by Dr. Michael Rosenblum).
Phase III studies: we provided a general framework for adaptive enrichment designs with delayed outcome in Phase III studies, using semiparametric locally efficient estimators at each interim analysis to leverage information in prognostic baseline variables and short term outcomes. We showed, through simulations of a real trial, that the adaptive design with the proposed estimator substantially reduced the sample size needed/expected to conduct a trial, yet has comparable power, bias, variance and mean squared error to standard design/estimators (Advised by Dr. Michael Rosenblum).
Phase III studies: we proposed a general prediction method for the identification of treatment covariate interactions (treatment response heterogeneity), as a secondary analysis in Phase III studies (Advised by Dr. Ravi Varadhan).
Seamless Trial Design: we constructed an adaptive seamless Phase I/II/III trial design in oncology, joint work with Dr. Alan Chiang and Dr. Yong Lin from Eli Lilly.
Wearable Computing
I am working with Dr. Vadim Zipunnikov to construct novel physical disability score based on actigraphy profiles for real-time monitoring patients recovery.
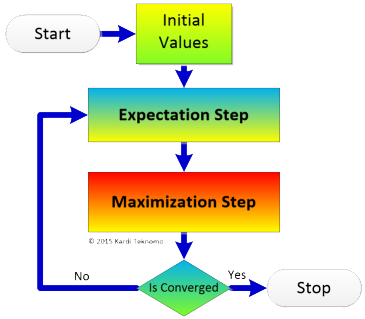
EM Algorithm Acceleration
I am working with Dr. Ravi Varadhan to study the acceleration of EM, MM and Other EM-like monotone algorithms.
Selected Work
"An Adaptive Multi-Stage Phase I Dose-finding Design Incorporating Continuous Efficacy and Toxicity Data from Multiple Treatment Cycles." International Chinese Statistical Association Conference (ICSA), Shanghai, China. Dec 19, 2016
"Optimal Two-Stage Adaptive Enrichment Design for Patient Population Recommendation Based on an Ordinal Risk Score in Planning Phase." Individualized Health Initiative(InHealth) Methodology Group Meeting, Johns Hopkins University, Baltimore. Nov 12, 2015
"Optimal Two-Stage Adaptive Enrichment Design for Patient Population Recommendation Based on an Ordinal Risk Score in Planning Phase." Joint Statistical Meeting(JSM 2015) Topic-Contributed Session, Seattle. August 11, 2015
Rosenblum, M., Qian, T., Du, Y., Qiu, H., and Fisher, A. Multiple Testing Procedures for Adaptive Enrichment Designs: Combining Group Sequential and Reallocation Approaches. Biostatistics, 17(4), 650-662.